Are you frustrated by outcomes in Breast Conserving Surgery?
Breast conserving surgery is unsuccessful in removing the entire tumor 20% of the time, resulting in a positive tumor margin upon pathology analysis. This typically means the woman will need to undergo additional surgery.
A new clinical trial is studying whether the investigational Breast Cancer Locator System used during surgery can improve a surgeon’s ability to reduce positive margins.
About the Breast Cancer Locator Trial
The Breast Cancer Locator Trial is a prospective, multicenter, 1:1 randomized, controlled pivotal trial of the Breast Cancer Locator System used to guide partial mastectomy for breast cancer compared to traditional wire localization.
Target Enrollment:
452 Women
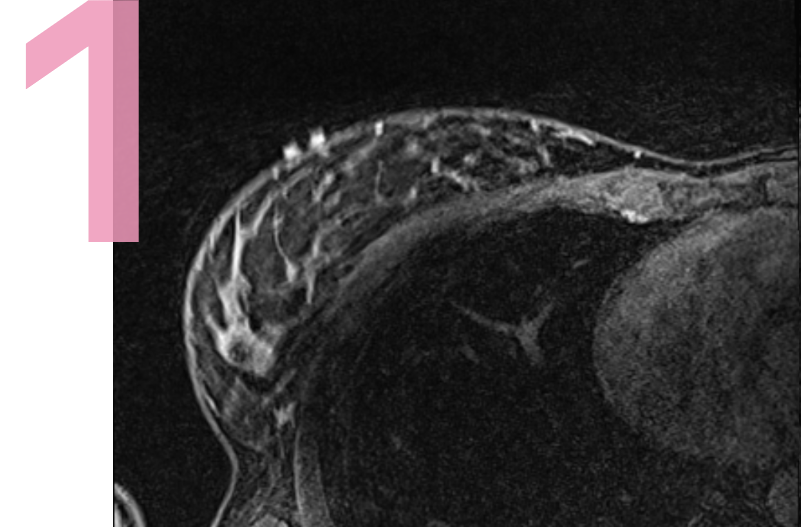
30 U.S. Centers
Endpoints
Primary Endpoint
- Positive margin rate in comparison to traditional wire localization
Other Endpoints
- Specimen volumes
- Rate of additional shave biopsies
- Re-excision rate
- Cancer localization rate
- Operative time
- Cost of care
- Adverse events
Who is the right subject?
Key inclusion criteria
- Women >18 years
- Histologic diagnosis of invasive breast cancer or DCIS
- Non-palpable, unifocal tumor or multifocal tumor with satellite lesions ≤ 2cm from primary tumor
- Tumor ≥ 0.8 cm in diameter visible on prone breast MRI imaging
Key exclusion criteria
- Absolute contraindication to MRI, including presence of implanted medical device (e.g., pacemaker or neurostimulator), aneurysm clip, or metallic foreign body in or near eyes
- Contraindication to use of gadolinium-based intravenous contrast, including life-threatening allergy
- Severe claustrophobia that precludes prone or supine MRI
- Compromised renal function, including chronic, severe kidney disease or acute kidney injury
- Pregnancy
- Previous or planned neoadjuvant chemotherapy
- Sternal notch-to-nipple distance of > 32cm
- > 135cm circumference around breasts and arms for sites using 60cm bore scanners, and > 145 cm for sites using 70cm bore scanners
- Known allergy to materials in device
- Use of localization with devices other than a localization wire
- Would require > 2 localization wires, if randomized to standard of care arm
- Multicentric tumors (additional tumors > 2cm from primary tumor)
- Would require chest wall muscle nerve block as part of the operation
About the Breast Cancer Locator

Richard Barth, Jr., MD
Chief of General Surgery, Dartmouth-Hitchcock Medical Center
Professor of Surgery, Geisel School of Medicine at Dartmouth
Co-founder, CairnSurgical (developer of the Breast Cancer Locator)
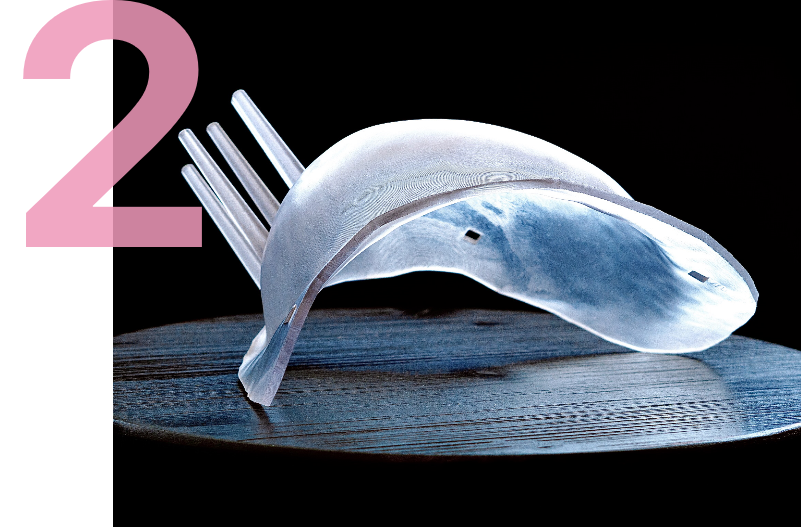
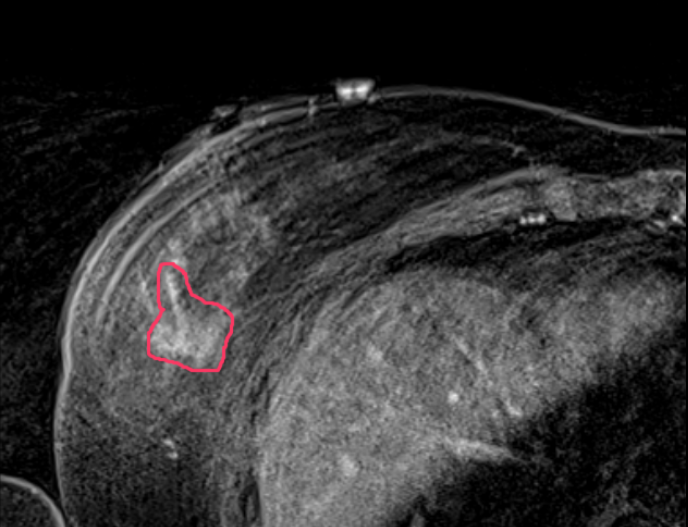
A supine MRI image is first performed with the breast positioned in its surgical position providing precise detail on the size, shape, depth, edges and 1 centimeter margin for the tumor. This imagery is used to analyze the tumor and construct a three-dimensional tumor model that serves as the basis for two surgical tools.
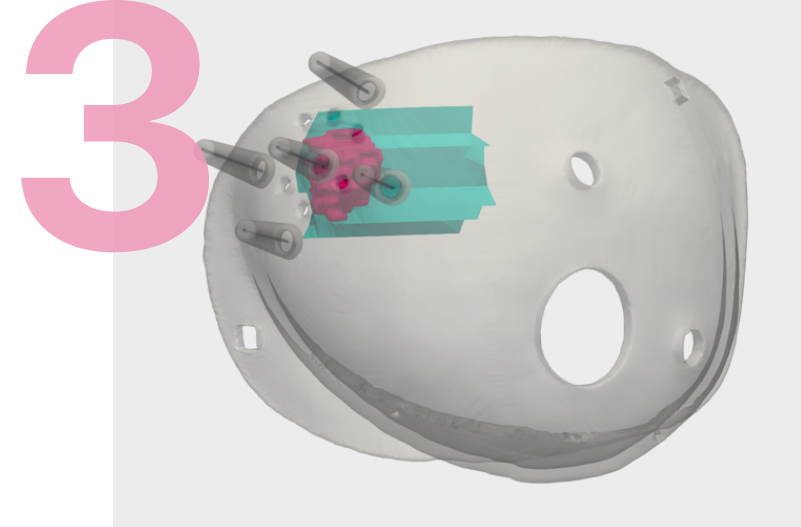
Participating Centers
St. Joseph Hospital Rehabilitation
2300 Southwood Drive
Nashua, NH 03063
Ph: 603.577.4171
Bedford Medical Park
9 Washington Place
Bedford, NH 03110
Ph: 603.663.5270
104 Endicott Street, Suite 200
Danvers, MA 01923
Ph: 978.882.6899
Breast Surgical Oncology
1400 Pressler Unit 1434
Houston, TX 77030-4008
Ph: 713.792.5031
1301 Palm Avenue
Jacksonville, FL 32207
Ph: 904.202.7070
A Division of Arizona Center for Cancer Care
9965 N. 95 th
St, Suite 105
Scottsdale, AZ 85258
Ph: 480.278.8261
101 Dudley Street
Providence, RI 02905
Ph: 401.453.7540
Dartmouth-Hitchcock Medical Center
One Medical Center Drive
Lebanon, NH 03756-3500
Ph: 603.650.4344
580 Court St
Keene, NH 03431
Ph: 603.354.5469
12902 USF Magnolia Drive
Tampa, FL 33612
Ph: 813.745.4673
317 South Manning Boulevard, Suite 220
Albany, New York 12208
Ph: 518.525.6739
Pond St
London NW3 2QG,
United Kingdom
Ph: +44.20.7794.0500
455 Toll Gate Rd
Warwick, RI 02886
Ph: 401.737.7000
1 st Floor NOWGEN Centre
Nightingale Centre Southmoor Road
Manchester M239LT
0300 33 9444
University Health Network – PMH
610 University Ave, 3-130
Toronto, Ontario Canada, M5G 2M9
Ph: 416.946.2292
701 Park Ave
Minneapolis,
MN 55415
Ph: 612.873.5300
860 Washington St
Boston,
MA 02111
161 Fort Washington Avenue
New York, NY 10032
Ph: 212.305.9676
460 W. 10th Avenue
Columbus, OH 43212
Ph: 614.293.4040
150 Park Ave
Florham Park, NJ 07932
Ph: 970.404.9945
8081 Innovation Park Drive
Fairfax, VA 22031
Ph: 571.506.8252
For Subjects


Breast conserving surgery is successful about 80 percent of the time in removing the entire tumor. However, one in five women with breast cancer must undergo a second surgery when some of the cancer is missed.
According to a recent study on breast cancer tumor shapes, fewer than 20 percent of breast cancer tumors are round. When the shape is irregular, it can be challenging for surgeons to easily identify its edges in order to remove all of it.
A new clinical trial is studying whether the investigational Breast Cancer Locator System used during surgery can improve a surgeon’s ability to reduce positive margins.

CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use
